| Title | Data from: Quantitative assessment of Zirconium-89 labeled cetuximab using PETCT imaging in patients with advanced head and neck cancer - a theragnostic approach |
| Publication Type | Dataset |
| Year of Publication | 2016 |
| Authors | Even, A, Hamming-Vrieze, O, van Elmpt, W, Winnepenninckx, V, Heukelom, J, Tesselaar, M, Vogel, W, Hoeben, A, Zegers, CML, Vugts, D, Van Dongen, G, Bartelink, H, Mottaghy, F, Hoebers, F, Lambin, P |
| Publication Language | eng |
| Keywords | cetuximab, EGFR, Immuno-PET, LAHNSCC, zirconium-89 |
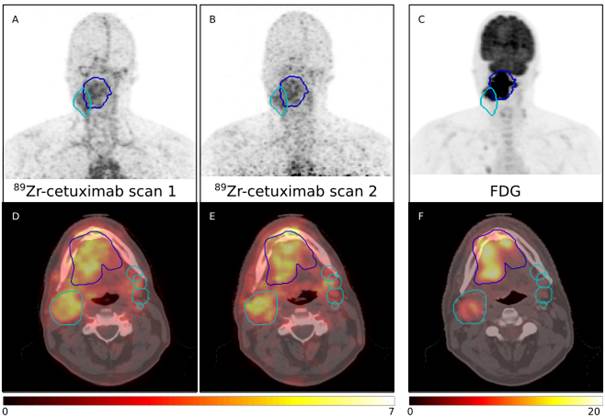
| Abstract | Biomarkers predicting treatment response to the monoclonal antibody cetuximab in locally advanced head and neck squamous cell carcinomas (LAHNSCC) are lacking. We hypothesize that tumor accessibility is an important factor in treatment success of the EGFR targeting drug. We quantified uptake of cetuximab labeled with Zirconium-89 (89Zr) using PET/CT imaging. Examples scans for four patients are provided. All patients received a planning PET/CT and two 89Zr-cetuximab scans. Patient P0037C0006I4475579 and P0037C0006I5879176 underwent 89Zr-cetuximab scans at 4 and 7 days post-injection; patient P0037C0006I6042760 and P0037C0006I8991415 at 3 and 6 days post-injection. All 89Zr-cetuximab PET scans are corrected for attenuation, scatter and 89Zr decay.
|
| DOI | 10.17195/candat.2016.11.1 |
| Original Publication | http://doi.org/10.18632/oncotarget.13910 |
| Attachment | Size |
|---|---|
| 97.26 MB | |
| 131.21 MB | |
| 115.71 MB | |
| 101.03 MB |